1. Understanding Sarcomas and Their Types
Sarcomas are categorized based on the tissue from which they originate. They can be broadly divided into soft tissue sarcomas and bone sarcomas. Soft tissue sarcomas develop in the soft tissues such as fat, muscle, and connective tissues, while bone sarcomas arise in the bones. Within these categories, there are several subtypes, including:
- Osteosarcoma: Originating in the bones, commonly affecting adolescents.
- Liposarcoma: Arising in fat tissue, usually seen in adults.
- Leiomyosarcoma: Developing from smooth muscle tissue, found in organs like the uterus or digestive tract.
- Rhabdomyosarcoma: A rare form affecting skeletal muscle.
- Angiosarcoma: Originating in blood vessels, these can develop in any tissue with blood vessels.
Despite these variations, all sarcomas share the characteristic ability to invade local tissues and metastasize to distant organs.
2. Initial Growth and Local Invasion
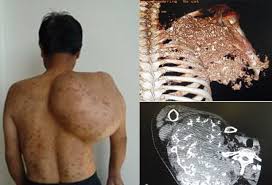
Sarcomas typically begin as a single mass in the affected tissue. Early on, they are confined to the primary site, but as the tumor grows, it can begin to invade surrounding tissues. The initial phase of growth involves the accumulation of genetic mutations that drive the uncontrolled division of cells. These mutations can alter key genes responsible for cell growth, apoptosis (programmed cell death), and tissue repair. This leads to the formation of a tumor with increased cellular proliferation and reduced ability to undergo apoptosis, allowing the tumor to persist and grow.
As the tumor enlarges, it exerts physical pressure on nearby tissues, which can lead to inflammation, pain, and dysfunction of the surrounding structures. Some sarcomas produce enzymes called matrix metalloproteinases (MMPs) that degrade the extracellular matrix, enabling the cancer cells to invade surrounding tissues. These enzymes are critical for the tumor to breach the basement membrane and invade neighboring tissues, a critical step in the spread of sarcomas.
3. Lymphatic and Hematogenous Spread
While sarcomas initially grow locally, their ultimate danger lies in their ability to spread to distant organs. This process is known as metastasis, and sarcomas can metastasize in one of two primary ways: lymphatic spread and hematogenous (blood) spread.
3.1 Lymphatic Spread
Lymphatic spread occurs when sarcoma cells invade the lymphatic system, which is part of the body’s immune system that helps fight infections and maintain fluid balance. The lymphatic system consists of lymph nodes, vessels, and fluid. Once tumor cells invade the lymphatic vessels, they can travel through the lymphatic channels and eventually lodge in regional or distant lymph nodes. This spread is less common in sarcomas compared to carcinomas but is still a significant route for some sarcoma subtypes.
Lymph node involvement is often an indicator of disease progression and can affect treatment strategies. For example, if sarcoma cells have spread to the lymph nodes, surgical removal of these nodes might be required in conjunction with other therapies such as radiation or chemotherapy. However, not all sarcoma subtypes spread through the lymphatic system, and some may bypass the lymph nodes altogether.
3.2 Hematogenous Spread
Hematogenous spread, or the spread through the bloodstream, is the most common method by which sarcomas metastasize to distant organs. Tumor cells invade blood vessels, typically veins, and are carried away from the primary tumor site to other parts of the body through circulation. Once these cells reach distant organs, such as the lungs, liver, or bones, they can settle, establish secondary tumors, and begin growing in a new location.
The lungs are the most frequent site for hematogenous metastases in sarcoma patients, particularly in cases of osteosarcoma, leiomyosarcoma, and rhabdomyosarcoma. Other common sites of metastasis include the liver, bones, and, less commonly, the brain or other organs. The ability of sarcoma cells to establish secondary tumors in these organs is influenced by several factors, including the tumor’s molecular characteristics, the vascularity of the organ, and the ability of the metastatic cells to adapt to the new environment.
4. The Role of Angiogenesis in Metastasis
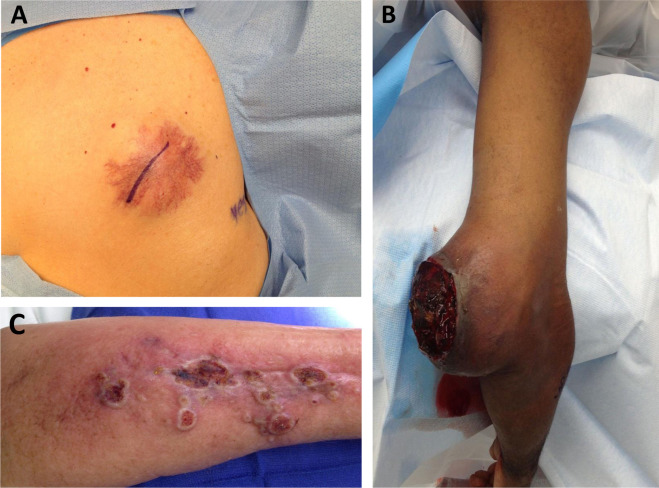
Angiogenesis, the formation of new blood vessels, plays a crucial role in the metastatic process. For a tumor to grow beyond a small size, it needs a sufficient blood supply to deliver oxygen and nutrients. As sarcomas enlarge, they can stimulate angiogenesis through the release of signaling molecules such as vascular endothelial growth factor (VEGF). VEGF promotes the growth of new blood vessels, which enables the tumor to receive the nutrients and oxygen necessary for continued growth.
However, this new blood supply also provides a route for sarcoma cells to enter the bloodstream, allowing for hematogenous spread. The creation of new blood vessels is not only essential for tumor growth but also increases the likelihood that cancer cells will travel to distant organs, where they can establish secondary growths.
5. The Metastatic Cascade
The process of metastasis is complex and involves multiple steps, known as the metastatic cascade. These steps include:
- Invasion: Tumor cells invade surrounding tissues, including the extracellular matrix and blood vessels, often through the action of enzymes like matrix metalloproteinases.
- Intravasation: Tumor cells enter blood vessels or lymphatic vessels, where they travel through the circulatory system to distant organs.
- Survival in Circulation: Once in circulation, tumor cells must survive in the hostile environment of the bloodstream, where they are exposed to immune cells and other factors that could destroy them.
- Extravasation: Tumor cells exit the bloodstream and invade the tissue of distant organs. This is facilitated by the ability of cancer cells to adhere to the endothelial cells lining blood vessels and pass through the vessel walls.
- Colonization and Growth: After extravasation, the tumor cells must adapt to the new environment and form micrometastases. Over time, these small clusters of cancer cells can grow into clinically detectable secondary tumors.
The success of metastasis is influenced by both the intrinsic properties of the cancer cells and the microenvironment of the organs they are attempting to colonize. Some sarcoma cells are more likely to metastasize due to their ability to evade the immune system, avoid apoptosis, and survive in foreign tissue environments.
6. Molecular Mechanisms Involved in Metastasis
Several molecular mechanisms facilitate the metastasis of sarcomas. These include:
- Epithelial-Mesenchymal Transition (EMT): Although sarcomas are mesenchymal tumors by nature, they can undergo a form of EMT, where they acquire epithelial-like characteristics that allow them to invade surrounding tissues and migrate to distant sites.
- Cell Adhesion Molecules: Proteins like integrins and cadherins mediate the ability of tumor cells to stick to extracellular matrix components and blood vessel walls. Changes in these molecules can enhance the tumor’s invasive capacity.
- MicroRNAs and Gene Expression: Alterations in the expression of microRNAs and other regulatory genes can influence the tumor’s ability to invade tissues, survive in circulation, and colonize distant organs.
- Immune Evasion: Sarcoma cells can evade immune detection through various mechanisms, such as expressing immune checkpoint molecules (e.g., PD-L1), which inhibit immune cell activity.
These molecular changes are often targeted in experimental therapies that aim to block metastasis and improve treatment outcomes.
7. Diagnosis and Detection of Metastasis
Detecting metastasis in sarcoma patients is crucial for determining the stage of cancer and planning treatment. Various imaging techniques are used to detect metastatic spread, including:
- CT Scans and MRI: These imaging tools are highly effective for identifying the primary tumor and detecting spread to the lungs, liver, and other organs.
- PET Scans: Positron emission tomography (PET) scans can identify metabolically active metastatic lesions and are useful for monitoring treatment responses.
- Biopsy: In some cases, a biopsy of the metastatic site may be needed to confirm the presence of sarcoma cells and determine the type of sarcoma.
The staging of sarcoma, including the extent of metastasis, helps guide treatment decisions and provides important prognostic information.
8. Treatment of Metastatic Sarcoma
The treatment of metastatic sarcoma involves a multi-disciplinary approach and depends on the type of sarcoma, the extent of metastasis, and the patient’s overall health. Common treatment modalities include:
- Surgery: Surgical removal of the primary tumor and metastases, when feasible, is the most effective treatment for localized sarcoma and isolated metastatic lesions.
- Chemotherapy: Chemotherapy is often used to target sarcoma cells that have spread to distant sites. Drugs like doxorubicin and ifosfamide are commonly used for many types of sarcomas.
- Radiation Therapy: Radiation may be used to shrink or control metastatic tumors, especially in areas where surgery is not possible.
- Targeted Therapy: Newer therapies aim to target specific molecular pathways that contribute to tumor growth and metastasis.
- Immunotherapy: Immunotherapy, including immune checkpoint inhibitors, is a promising area of research for treating metastatic sarcoma by stimulating the patient’s immune system to attack cancer cells.
9. Prognosis and Survival Rates
The prognosis for patients with metastatic sarcoma depends on several factors, including the type of sarcoma, the extent of metastasis, and the response to treatment. While the survival rate for sarcoma patients has improved in recent years with advances in treatment, metastatic disease is still associated with a poor prognosis in many cases.
In general, sarcomas that metastasize to the lungs or other organs are challenging to treat and often have lower survival rates. However, some patients with isolated metastatic disease or those who respond well to treatment can achieve long-term survival.
Sarcomas are a diverse group of tumors with the potential to spread through the body via lymphatic and hematogenous routes. The process of metastasis involves a series of complex biological steps, from local invasion to colonization of distant organs. Understanding how sarcomas metastasize is critical for developing effective treatments and improving patient outcomes. Continued research into the molecular mechanisms of metastasis holds promise for the development of targeted therapies that can better manage metastatic sarcoma and potentially improve survival rates for affected patients.