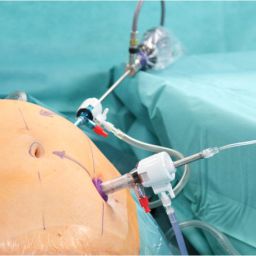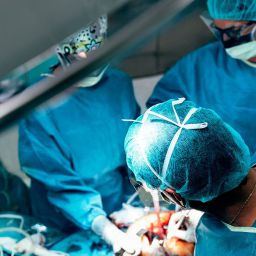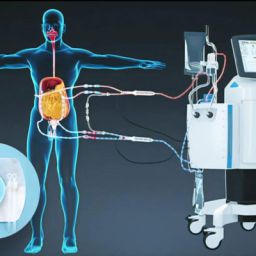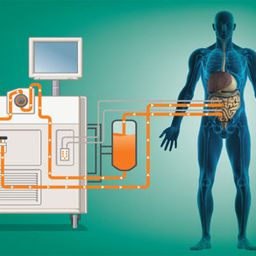
Hyperthermic Intraperitoneal Chemotherapy (HIPEC) is a groundbreaking technique in cancer treatment. It combines cytoreductive surgery (CRS) with the direct application of heated chemotherapy into the abdominal cavity, aiming to eradicate microscopic cancer cells left behind after surgery. This approach has shown promise, particularly in cancers confined to the peritoneal cavity, such as peritoneal carcinomatosis arising from colorectal, ovarian, gastric cancers, and mesothelioma. Understanding the role of HIPEC in preventing cancer spread involves exploring its mechanisms, applications, efficacy, and limitations.
This article delves deeply into how HIPEC prevents cancer progression, its current applications, and its potential future roles in oncology.
The Basics of HIPEC Therapy
How HIPEC Works
HIPEC involves two main stages:
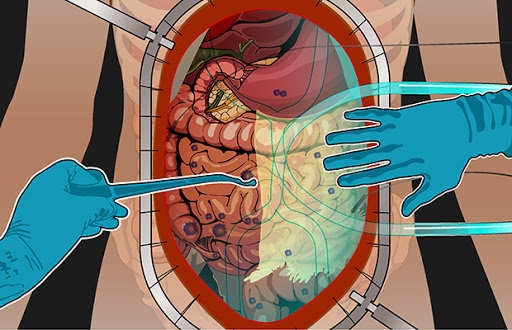
- Cytoreductive Surgery (CRS): Surgeons remove visible tumors from the abdominal cavity. This step is critical to reduce the tumor burden.
- HIPEC Procedure: After tumor removal, a heated chemotherapy solution is circulated within the abdominal cavity for 60–120 minutes. The chemotherapy agent targets residual microscopic cancer cells that remain undetectable during surgery.
The process benefits from:
- Localized Treatment: Direct delivery of chemotherapy to the peritoneum allows for high drug concentrations with limited systemic toxicity.
- Hyperthermia: Heating the solution to 41–43°C increases the effectiveness of chemotherapy by enhancing drug penetration, increasing cytotoxicity, and impairing cancer cell repair mechanisms.
Cancer Spread and the Peritoneal Cavity
Mechanisms of Peritoneal Cancer Spread
Peritoneal cancer spread often occurs in cancers of the gastrointestinal and gynecological systems. The mechanisms include:
- Seeding: Cancer cells shed from the primary tumor and implant themselves on peritoneal surfaces.
- Circulating Fluids: Peritoneal fluid distributes cancer cells throughout the abdominal cavity.
- Angiogenesis: The development of new blood vessels supports tumor growth and spread.
Challenges in Treating Peritoneal Metastases
Traditional systemic chemotherapy has limited penetration into the peritoneum due to the peritoneal-plasma barrier. This barrier prevents adequate drug concentrations in the peritoneal cavity, allowing microscopic residual cancer cells to persist and grow.
HIPEC offers a localized and targeted solution to these challenges, directly addressing cancer cells in the abdominal cavity.
Applications of HIPEC in Cancer Treatment
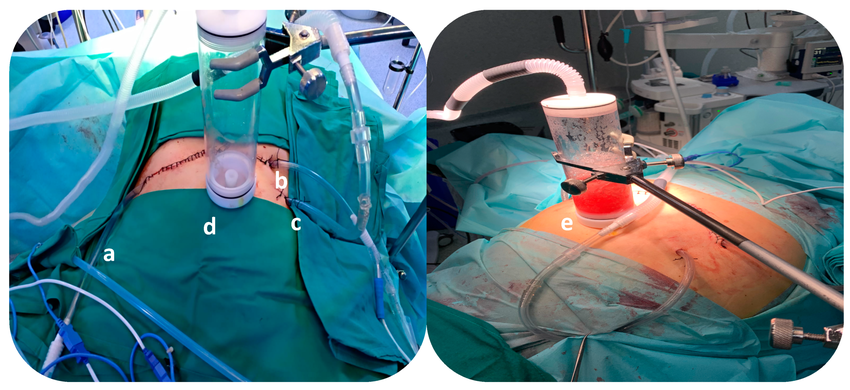
Colorectal Cancer
Colorectal cancer is a leading cause of peritoneal carcinomatosis. HIPEC has become a standard adjunct to CRS for select patients with peritoneal metastases from colorectal cancer. Studies show improved survival rates and reduced recurrence compared to surgery or chemotherapy alone.
Ovarian Cancer
Ovarian cancer often spreads within the peritoneal cavity. HIPEC, applied after interval cytoreduction, has been shown to improve progression-free and overall survival. It is increasingly used in advanced-stage ovarian cancer.
Gastric Cancer
Peritoneal dissemination is common in advanced gastric cancer. HIPEC has demonstrated potential in reducing peritoneal recurrence and improving survival in carefully selected patients.
Peritoneal Mesothelioma
Peritoneal mesothelioma is a rare but aggressive cancer originating in the peritoneum. HIPEC has shown to extend survival significantly when combined with complete cytoreduction.
Mechanisms of Action: How HIPEC Prevents Cancer Spread
Direct Cytotoxic Effects
HIPEC delivers chemotherapy directly to cancer cells within the peritoneum. The high concentration and prolonged exposure maximize the cytotoxic effects on residual microscopic disease.
Thermal Enhancement
Hyperthermia amplifies chemotherapy’s effectiveness by:
- Increasing drug uptake by cancer cells.
- Inhibiting DNA repair mechanisms, making cancer cells more susceptible to apoptosis.
- Reducing cancer cell resistance to chemotherapeutic agents.
Reduced Systemic Toxicity
Since HIPEC confines chemotherapy to the abdominal cavity, it minimizes systemic side effects, allowing the use of higher doses of chemotherapy.
Prevention of Recurrence
By targeting residual cancer cells that would otherwise lead to recurrence, HIPEC serves as a critical step in achieving long-term disease control.
Clinical Evidence Supporting HIPEC
Survival Benefits
Numerous studies have highlighted HIPEC’s role in improving survival. For example:
- In colorectal cancer with peritoneal carcinomatosis, the combination of CRS and HIPEC has demonstrated median survival rates of 30–40 months, compared to 12 months with systemic therapy alone.
- In ovarian cancer, randomized trials have shown that HIPEC significantly extends progression-free survival compared to conventional treatments.
Recurrence Reduction
HIPEC reduces peritoneal recurrence rates by eradicating microscopic disease, particularly in cancers prone to peritoneal dissemination, such as gastric and ovarian cancers.
Quality of Life
Despite its intensive nature, HIPEC has been associated with an acceptable quality of life for patients, particularly when performed at experienced centers.
Limitations and Challenges
Patient Selection
Not all patients are candidates for HIPEC. Strict selection criteria include:
- Limited disease burden.
- Good overall health and performance status.
- Complete or near-complete cytoreduction achievable during surgery.
Procedural Risks
HIPEC is associated with complications, such as:
- Postoperative infections.
- Bowel perforations.
- Chemotherapy-induced side effects like nephrotoxicity and myelosuppression.
Lack of Universal Guidelines
HIPEC protocols vary widely among institutions, including differences in chemotherapy agents, dosages, and heating methods. This variability complicates the standardization of treatment.
Future Directions for HIPEC
Enhanced Chemotherapy Agents
Ongoing research focuses on developing more effective chemotherapy drugs specifically designed for intraperitoneal use.
Immunotherapy Integration
Combining HIPEC with immunotherapy holds promise for enhancing the immune system’s ability to combat residual cancer cells.
Personalized Medicine
Advancements in genomic profiling may allow for tailored HIPEC protocols based on individual tumor biology, improving outcomes and minimizing toxicity.
Expanded Indications
Researchers are exploring HIPEC’s use in other cancers, such as pancreatic cancer, to expand its therapeutic reach.
HIPEC represents a significant advancement in the treatment of peritoneal carcinomatosis and other cancers with a propensity for peritoneal spread. By directly targeting residual cancer cells within the peritoneal cavity, HIPEC not only reduces recurrence but also enhances survival and quality of life for carefully selected patients. While challenges remain, including procedural risks and the need for standardization, ongoing research promises to expand HIPEC’s role in cancer therapy. Early detection, precise patient selection, and integration with novel therapeutic approaches will further solidify HIPEC’s place in oncology.
This innovative therapy is a beacon of hope for patients with advanced cancers, transforming a historically devastating prognosis into a more manageable condition with meaningful survival benefits.